May 26, 2020
- Wireless wearable biosensor (Philips Biosensor BX100) receives 510(k) clearance from the FDA and CE mark to help monitor COVID-19 patients in hospital, with first install at OLVG Hospital in the Netherlands
- Further enhances Philips portfolio of devices, software and services for identifying patients at risk for deterioration while limiting exposure to help improve staff and patient safety and preserve valuable personal protective equipment
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its wearable biosensor (Philips Biosensor BX100) to help manage confirmed and suspected COVID-19 patients in the hospital. The next generation wireless wearable biosensor enhances clinical surveillance in the Philips patient deterioration detection solution to help clinicians detect risk so they can intervene earlier and help improve care for patients in lower acuity care areas. The solution has already received CE mark, and is currently in use with the first install at the OLVG Hospital in the Netherlands to help manage the triage and clinical surveillance of COVID-19 patients.
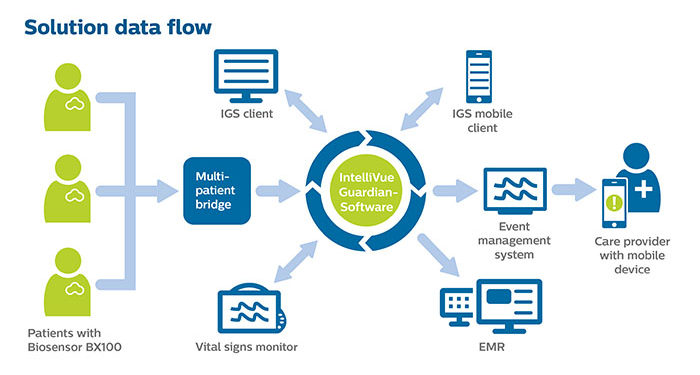
The Philips Biosensor BX100 is designed to address a new approach to vital signs measurements, supporting surveillance of higher acuity patients moving from intensive care units into lower acuity general care areas of a hospital. The lightweight, disposable biosensor is a 5-day, single-use wearable patch which can be integrated with a scalable hub to monitor multiple patients across multiple rooms. Built to incorporate into existing clinical workflows for mobile viewing and notifications, the device requires no cleaning or charging. The medical-grade wireless wearable biosensor, intended for use by healthcare professionals on patients 18 years of age and older, adheres discreetly to the chest to collect, store, measure and transmit respiratory rate and heart rate every minute – the top two predictors of deterioration – as well as contextual parameters such as posture, activity level and ambulation.
OLVG, a top clinical, referral and training hospital in the Netherlands is responding to the COVID-19 emergency situation by remotely monitoring patients in isolation rooms who are diagnosed or suspected of COVID, but don’t need ventilation. To meet the hospital’s need to support COVID-19 patients in isolation, OLVG has implemented Philips patient deterioration detection solution comprised of data-driven intelligent analytics software (IntelliVue GuardianSoftware) for early warning scoring, advanced patient monitors (EarlyVue VS30), and the Philips Biosensor BX100 wearable sensors.
During this unprecedented time of COVID-19, the Philips Biosensor BX100 helps provide rapid deployment for clinical surveillance to help decrease risk of exposure of healthcare workers while acquiring frequent patient vitals, and easing the demand for personal protective equipment (PPE).
Peter Ziese
General Manager Monitoring and Analytics at Philips.
“During this unprecedented time of COVID-19, the Philips Biosensor BX100 helps provide rapid deployment for clinical surveillance to help decrease risk of exposure of healthcare workers while acquiring frequent patient vitals, and easing the demand for personal protective equipment (PPE),” said Peter Ziese, General Manager Monitoring and Analytics at Philips. “The biosensor is an integral component in our Patient Deterioration Detection solution which helps aid in the identification of the subtle signs of deterioration in a patient’s condition at the point of care, hours before a potential adverse event would occur.”
“With the help of this new biosensor, we can continuously and remotely monitor patients, which is especially important on the COVID-19 wards,” said Florian van der Hunnik, Chief Nursing Information Officer and team leader of the COVID-19 ward at OLVG Hospital in Amsterdam. “Because we cannot walk in and out of the patient rooms without protective gear, we welcome this innovation as it helps improve how we can do our jobs better.”