Jul 03, 2019
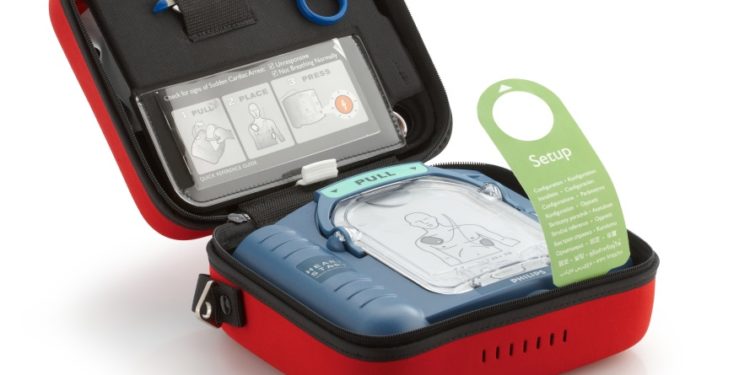
Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced shipment of its two-millionth AED (Automated External Defibrillator), which was delivered to a customer in Italy. This achievement marks a major milestone in almost 20 years of Philips innovation in cardiac resuscitation aimed at providing personalized therapy to victims of sudden cardiac arrest. Cardiac arrests can happen anywhere, at any time, and bystander intervention and treatment with an AED has been shown to triple the survival rate to 31.4 percent [1].
“We are very proud of the knowledge that Philips AEDs have helped to save lives every day for almost two decades,” said Arman Voskerchyan, Business Leader Therapeutic Care at Philips. “As we have reached the incredible milestone of shipping our two-millionth AED worldwide, we will continue to provide our customers with reliable, easy-to-use lifesaving solutions.”
We are very proud of the knowledge that Philips AEDs have helped to save lives every day for almost two decades.
Arman Voskerchyan
Business Leader Therapeutic Care at Philips.
A pioneer in AED innovation and reliability
Philips’ HeartStart OnSite AED made the implementation of early AED programs in communities, schools and businesses much easier, while the Philips HeartStart Home AED remains the only AED available in the US for home use. Philips was also the first to introduce an AED for pediatric use, and was a pioneer in providing AEDs for use in airplanes. Today, Philips AEDs are available across the globe, on board major airlines, in Fortune 100 companies, and in the locker rooms of professional sports teams worldwide. Philips HeartStart Home and OnSite AEDs shipped since January 2013 have a reliability record of approximately 99.9% per year.
Philips recently announced that the US Food and Drug Administration (FDA) has approved the company’s premarket approval (PMA) application for its HeartStart OnSite AED [2] and HeartStart Home AED [3], and the relevant accessories, such as batteries and electrodes [4]