A new efficient alloy-based catalyst developed for improved hydrogen production through electrolysis of water into hydrogen and oxygen, can pave the path towards a solution for clean energy production.
This innovative approach using high-entropy alloy (HEA), could reduce reliance on expensive materials like platinum for clean energy production.
Typically, alloys are metallic substances composed of two or more elements, that are prepared by adding relatively small amounts of secondary elements to a primary metal. High Entropy Alloys (HEAs), on the other hand, are advanced materials that contain multiple elements (usually five or more) in almost equal concentrations. Here, the entropic (state of disorder) contribution to the total free energy overcomes the enthalpic (sum of internal energy and the product of its pressure and volume) contribution and, thereby, stabilizes the alloy formation. These HEAs are known for their versatility and potential to replace commercial catalysts in water-splitting applications. In this context, preparation of single-phase HEA nanoparticles devoid of any impurity phases by bottom-up chemical synthetic methods is highly challenging.
Researchers at the Centre for Nano and Soft Matter Sciences (CeNS) in Bengaluru, an autonomous institute of the Department of Science and Technology (DST) have developed a novel high-entropy alloy (HEA) catalyst called PtPdCoNiMn (consisting of Platinum, Palladium, Cobalt, Nickel and Manganese). The selection of these constituent metals was based on guidelines designed and developed by Dr. Prashant Singh, a Staff Scientist from AMES National Laboratory, USA. Once the final composition was identified, the CeNS researchers prepared the HEA via two different approaches – electrodeposition at room temperature and atmospheric pressure and (chemical synthesis under high temperature and pressure in a given solvent called solvothermal processes.
For the electrodeposition, the choice of solvent and the deposition potential was optimized for developing the HEA. In the solvothermal method, through a series of optimization steps the researchers carefully selected the right solvent and reducing agent in precise ratios to control the reaction rate and synthesis process. These methods allowed the production of alloys with two, three, four, or all five elements in either single-phase or multi-phase forms. The PtPdCoNiMn HEA catalyst, created by combining platinum (Pt), palladium (Pd), cobalt (Co), nickel (Ni), and manganese (Mn), resulted in efficient hydrogen production with minimal energy loss, high durability, and long-term stability. Theoretical studies indicated the optimal binding of reaction intermediates on the catalyst surface to be the reason for the superiority of the developed HEA over commercial catalysts for hydrogen generation.
As the HEA catalyst used seven times less platinum than commercial catalyst and offered better catalytic efficiency than pure platinum, it could be a viable alternative to conventional catalysts. These HEAs also displayed good performance in practical settings, including alkaline seawater, maintaining stability and efficiency for over 100 hours without degradation.
This advancement could pave the way for cleaner, more affordable hydrogen production, benefiting industries and renewable energy technologies. The research was funded by India’s Anusandhan National Research Foundation (ANRF), of which the Department of Science and Technology (DST) is the administrative Department. Two papers from the research were recently published in the journals Advanced Functional Material and Small.
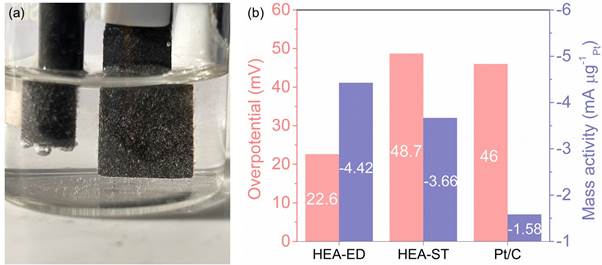
Figure a) Hydrogen generation from HEA electrodeposited on carbon paper in a three-electrode system. Figure b) A comparison plot of the hydrogen generation performance of electrodeposited HEA (HEA-ED), HEA prepared using solvothermal method (HEA-ST) and commercial Pt/C.

From L to R: Dr. Ashutosh Singh, Prof. B. L. V. Prasad and Ms. Athira Chandran.